The design of artificial reefs influences the ecological fate of restored reefs

Ewout Knoester’s team in Kenya has shown that the type of artificial reef structure used for coral restoration has an impact on the survival of corals and their diversity, but also on the diversity of fish and other animal species in the coral ecosystem. The combined use of metal cages and layers of concrete appears to be the most optimal design.
Worldwide coral reef cover is steadily declining (Eddy et al., 2021), notably due to massive bleaching events (Ainsworth and Brown, 2021). There are other, more local causes, such as overfishing (Mumby and Harborne, 2010), nautical activities (Dinsdale and Harriott, 2004; Flynn and Forrester, 2019), increased predation by crown of thorns Acanthaster spp. starfish (Kayal et al., 2012), etc. The causes of coral reef mortality are often multifactorial (Sale, 2008). Prior to any coral reef restoration effort, it is important to understand the cause(s) of reef mortality and to counter them as much as possible, then to protect the reef zone being restored (Hughes et al., 2007; Mumby and Harborne, 2010). Once this has been done, there are several choices to be made about the restoration methods to be implemented, and the use of artificial reefs as substrate support is a widespread option. But few studies compare different artificial reef structures, and this is what Ewout Knoester and his team have done over two years (Knoester et al., 2023).
Artificial reefs tested
The artificial reefs were placed in Kenya, in the waters around the village of Mkwiro (Figure 1A). In addition to a control zone without corals (Figure 1B), and a reference zone with an unrestored reef (Figure 1C), Knoester et al (2023) tested several artificial reefs:
- 16 modules each consisting of 8 glass bottles fixed to a concrete disc (figure 1D),
- 4 metal cages (figure 1E),
- 8 stacks of 4 concrete discs separated by PVC tubes (figure 1F),
- a mix of 4 glass bottle modules, 1 cage and 2 concrete stacks (figure 1G).
Each artificial reef was reproduced 10 times, giving a total of 40 artificial reefs tested, along with 10 control points and 10 reference points.
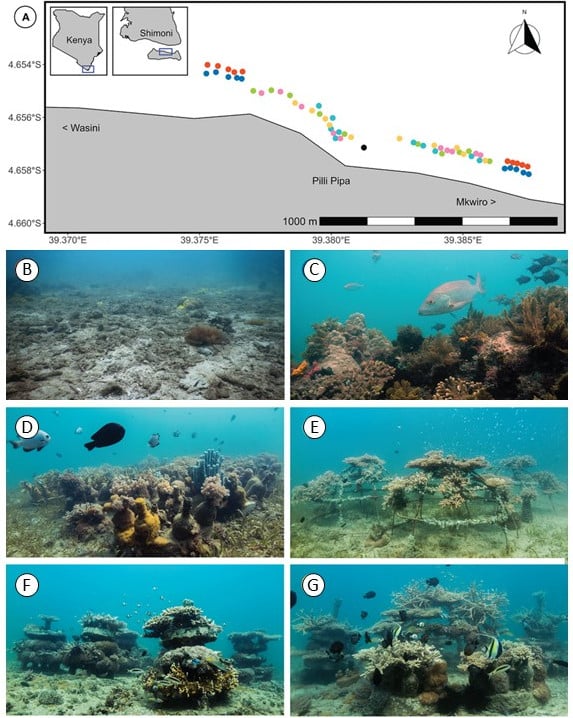
Figure 1: Map of the study area in Kenya (A) and photos at the start of the experiment of the control area without reef (B), the reference reef area (C), the artificial reef made of glass bottles (D), the artificial reef made of cages (E), the artificial reef made of concrete discs (F) and the mixed artificial reef (G).
Results
Although the hard coral cover of artificial reefs was similar to that of reference reefs, except for bottle reefs, coral diversity was much lower in artificial reefs (Figure 2), with a very strong dominance of Acropora spp. corals, which are fast-growing branching corals. Coral survival was 60% after two years, with variability in the causes of mortality linked to the type of reef. The survival rate was better in metal cages, because there was no predation by Acanthaster spp. starfish or breakage by tortoises rubbing against the corals. Conversely, bottle reefs have a higher mortality rate for these reasons.
Although corals have a better survival rate in cages, recruitment is much lower than in bottles or concrete stacks. Recruitment is always better, in terms of numbers and diversity, on reference reefs. The limited surface area available on the cages explains this difference. It also seems that concrete is a surface that allows coral larvae to attach.
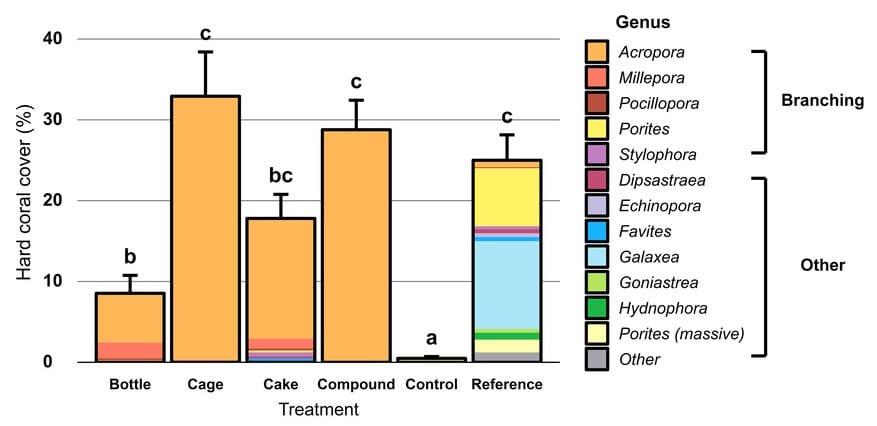
Figure 2: Percentage cover of hard corals (including Millepora hydrozoa) after two years of coral restoration. The error bar corresponds to the SE (n = 10). Treatments with the same letters do not differ significantly (p<0.05). Colours represent coral genera, divided between branching corals and other coral forms.
In terms of ecological and functional diversity, the reference reefs are always more diverse than the artificial reefs, but the latter show interesting and significant results. Fish are just as numerous in terms of biomass and abundance in the artificial or reference reefs, with a greater diversity of species in the restored reefs. There were no differences between the artificial reefs in terms of fish communities, with hard coral cover appearing to be more of a determining factor than the type of artificial reef. In addition, all trophic levels are present: the functional diversity required for a reef to function ecologically is therefore present in every artificial reef.
On the other hand, invertebrate diversity depends on the groups considered, but it is always higher on control reefs. There are major differences between artificial reefs: cages have few invertebrates apart from gastropods, whereas bottles and disc stacks attract more invertebrates, although they never reach the diversity of reference reefs.
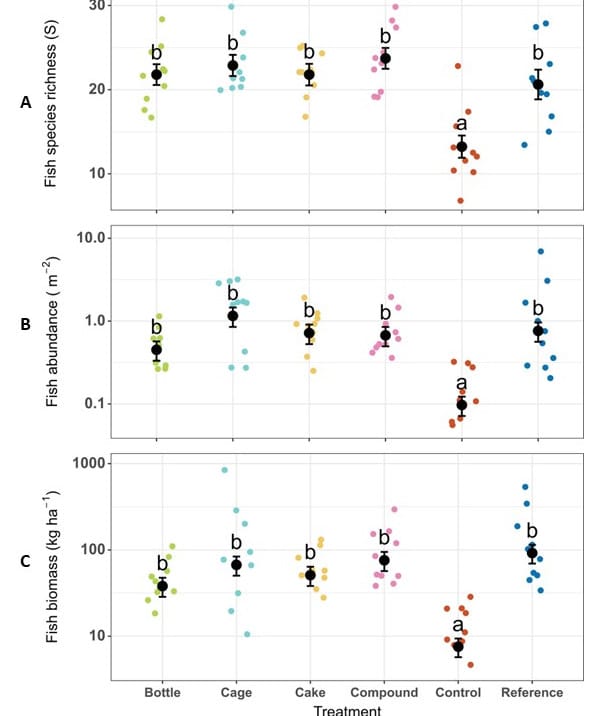
Figure 3: A – Fish species richness, B – Fish abundance and C – Fish biomass after two years of coral restoration. The error bar corresponds to the SE (n = 10). Treatments with the same letters do not differ significantly (p<0.05). The coloured points correspond to the different replicates for each treatment.
Conclusion
Although this study, conducted over 2 years, is a little too short to provide a long-term view of the evolution of restored reefs, it nevertheless provides some very interesting leads. In addition to the choice of location (Edwards and Clark, 1999) and the propagation technique, with or without nursery (Boström-Einarsson et al., 2020), the choice of artificial reef has an impact on the ecological recovery of the reefs. It would appear that a mix of metal cages and stacked concrete blocks offers a good compromise between the advantages and disadvantages of each. While the use of glass bottles does not seem to be the best solution for restoring the reef, it can create a den for turtles, which can rub against it.
References
Ainsworth, T.D., Brown, B.E., 2021. Coral bleaching. Curr. Biol. 31, R5–R6. https://doi.org/10.1016/j.cub.2020.10.048
Boström-Einarsson, L., Babcock, R.C., Bayraktarov, E., Ceccarelli, D., Cook, N., Ferse, S.C.A., Hancock, B., Harrison, P., Hein, M., Shaver, E., Smith, A., Suggett, D., Stewart-Sinclair, P.J., Vardi, T., McLeod, I.M., 2020. Coral restoration – A systematic review of current methods, successes, failures and future directions. PLOS ONE 15, e0226631. https://doi.org/10.1371/journal.pone.0226631
Cisneros-Montemayor, A.M., Greer, K., Palomares, M.L.D., Bruno, J.F., Ota, Y., Cheung, W.W.L., 2021. Global decline in capacity of coral reefs to provide ecosystem services. One Earth 4, 1278–1285. https://doi.org/10.1016/j.oneear.2021.08.016
Dinsdale, E.A., Harriott, V.J., 2004. Assessing Anchor Damage on Coral Reefs: A Case Study in Selection of Environmental Indicators. Environ. Manage. 33, 126–139. https://doi.org/10.1007/s00267-003-3056-9
Edwards, A.J., Clark, S., 1999. Coral transplantation: A useful management tool or misguided meddling? Mar. Pollut. Bull. 37, 474–487. https://doi.org/10.1016/S0025-326X(99)00145-9
Flynn, R.L., Forrester, G.E., 2019. Boat anchoring contributes substantially to coral reef degradation in the British Virgin Islands. PeerJ 7, e7010. https://doi.org/10.7717/peerj.7010
Hughes, T.P., Bellwood, D.R., Folke, C.S., McCook, L.J., Pandolfi, J.M., 2007. No-take areas, herbivory and coral reef resilience. Trends Ecol. Evol. 22, 1–3. https://doi.org/10.1016/j.tree.2006.10.009
Kayal, M., Vercelloni, J., Loma, T.L. de, Bosserelle, P., Chancerelle, Y., Geoffroy, S., Stievenart, C., Michonneau, F., Penin, L., Planes, S., Adjeroud, M., 2012. Predator Crown-of-Thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLOS ONE 7, e47363. https://doi.org/10.1371/journal.pone.0047363
Knoester, E.G., Rienstra, J.J., Schürmann, Q.J.F., Wolma, A.E., Murk, A.J., Osinga, R. 2023. Community-managed coral reef restoration in southern Kenya initiates reef recovery using various artificial reef designs. Front. Mar. Sci. 10:1152106. https://doi.org/10.3389/fmars.2023.1152106
Mumby, P.J., Harborne, A.R., 2010. Marine reserves enhance the recovery of corals on Caribbean reefs. PLOS ONE 5, e8657. https://doi.org/10.1371/journal.pone.0008657
Sale, P.F., 2008. Management of coral reefs: Where we have gone wrong and what we can do about it. Mar. Pollut. Bull. 56, 805–809. https://doi.org/10.1016/j.marpolbul.2008.04.009
Ces articles pourraient vous intéresser
CALL FOR PROJECT PROPOSALS
For the development of a coral reef conservation programme in partnership with Coral Guardian. About Coral Guardian Coral Guardian is a French non-profit that was…
13 April 2023
Sponsor spotlight : Alban from W2P
Tell us a bit about yourself 🙂 I am Alban Taravello, producer and founder of W2P Production. I am passionate about diving, deep-sea observation and video….
21 March 2023
How does the COP15 to the Convention on Biodiversity affect coral reefs?
Following the UN Climate Change Conference of the Parties (COP27) in Sharm el-Sheikh, Egypt (see our short review here), another key UN conference focusing on…
23 February 2023
How can reef fish-derived nutrients help corals under temperature stress?
The staggering biodiversity encountered in tropical coral reefs primarily results from the mutualistic symbiosis corals developed with endosymbiotic algae* from the family Symbiodinaceae. Thanks to…
20 January 2023
